 |
ISO 15223- 1 Reference no. 5.1.1. (ISO 7000-3082) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Manufacturer |
Indicates the medical device manufacturer |
 |
ISO 15223- 1
Reference no. 5.1.2. |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Authorized Representative in the European Community |
Indicates the authorized representative in the European Community / European Union |
 |
ISO 20417
Reference no. 6.1.2 d
Swissmedic’s Medical Device Ordinance (MedDO) |
Authorised representative for Switzerland |
Swiss authorised representative |
Indicates the authorised representative in Switzerland |
 |
ISO 15223- 1
Reference no. 5.1.3. (ISO 7000-2497) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Date of manufacture |
Indicates the date when the medical device was manufactured |
 |
SO 15223- 1
Reference no. 5.1.4. (ISO 7000-2607) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Use-by date |
Indicates the date after which the
medical device is not to be used
iso_15223 Use-by date
iso_grs_7000_2607 Use by date |
 |
ISO 15223- 1
Reference no. 5.1.5. (ISO 7000-2492) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Batch code |
Indicates the manufacturer's batch code so that the batch or lot can be identified |
 |
ISO 15223- 1
Reference no. 5.1.6. (ISO 7000-2493) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Catalogue number |
Indicates the manufacturer's catalog number so that the medical device can be identified
ISO 15223 Catalogue number
ISO 7000 Catalog number |
 |
ISO 15223- 1
Reference no. 5.1.7. (ISO 7000-2498) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Serial number |
Indicates the manufacturer's serial number so that a specific medical device can be identified |
 |
ISO 15223- 1
Reference no. 5.1.8. (ISO 7000-3725) |
Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. |
Importer |
Indicates the entity importing the medical device into the locale |
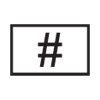 |
ISO 15223-1
Reference no. 5.1.10
(IEC 60417-6050) |
Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. |
Model number |
To identify the model number or type number of a product. In the application of this symbol, the model number or type number of the product should be accompanied with this symbol |
 |
ISO 15223-1
Reference no. 5.4.2. (ISO 7000-1051) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Do not reuse |
Indicates a medical device that is intended for one single use only
NOTE: Synonyms for “Do not reuse” are “single use” and “use only once”. |
 |
ISO 15223-1 Reference no. 5.4.3. (ISO 7000-1641) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Operator's manual; operating instructions |
Indicates the need for the user to consult the instructions for use
iso_15223 Consult instructions for use
iso_grs_7000_1641 Operator's manual; operating instructions |
 |
ISO 7000
Reference no. 2794 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
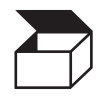
Packaging unit |
To indicate the number of pieces in the package. |
 |
ISO 15223-1, Reference no. 5.7.7
EU MDR GSPR 23.2 (q) |
Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied
Medical Device Regulation Annex 1 - General safety and performance requirements |
Medical device |
Indicates the item is a medical device. |
 |
ISO 15223-1 Reference no. 5.4.4. (ISO 7000-0434A) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Caution |
To indicate that caution is necessary when operating the device or control close to where the symbol is placed, or to indicate that the current situation needs operator awareness or operator action in order to avoid undesirable consequences |
 |
ISO 27185 -2011-05-09
ISO 7000 / IEC 60417 (ISO 7000-3079) |
Graphical symbols for use on equipment. |
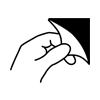
Open here |
To identify the location where the package can be opened and to indicate the method of opening it. |
 |
N/A |
N/A |
Open with Hand Symbol |
|
 |
EU 2017-745 EU 2017-746
Reference no. ANNEX V |
REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on medical devices, amending Directive 2001/83/ EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/ EEC and 93/42/EEC |
CE marking |
(43) ‘CE marking of conformity’ or ‘CE marking’ means a marking by which a manufacturer indicates that a device is in conformity with the applicable requirements set out in this Regulation and other applicable Union harmonisation legislation providing for its affixing. |
 |
UK Conformity assessed
UK MDR 2002 |
N/A |
UKCA marking |
|
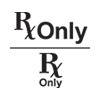 |
Code of Federal Regulations, Title 21, Part 801
21 CFR Part 801.1(c)(1)(i)F
21 CFR Part 801.109 |
N/A |
Prescription Use Only |
Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner. |
 |
ISO 15223-1
Reference no. 5.3.1. (ISO 7000-0621) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Fragile, handle with care |
Indicates a medical device that can be broken or damaged if not handled carefully |
 |
ISO 15223-1
Reference no. 5.3.2. (ISO 7000-0624) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Keep away from sunlight |
Indicates a medical device that needs protection from light sources |
 |
ISO 15223-1
Reference no. 5.3.4. (ISO 7000-0626) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Keep away from rain |
Indicates a medical device that needs protection from moisture
ISO 15223 Keep dry
ISO 7000 Keep away from rain |
 |
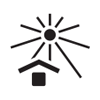
ISO 15223-1
Reference no. 5.3.5. (ISO 7000-0534) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Lower limit of temperature |
Indicates the lower limit of temperature to which the medical device can be safely exposed |
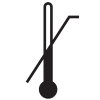 |
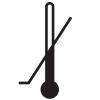
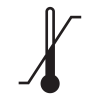
ISO 15223-1
Reference no. 5.3.6. (ISO 7000-0533) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Upper limit of temperature |
Indicates the upper limit of temperature to which the medical device can be safely exposed |
 |
ISO 15223-1
Reference no. 5.3.7. (ISO 7000-0632) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Temperature limit |
Indicates the temperature limits to which the medical device can be safely exposed |
 |
ISO 15223-1
Reference no. 5.2.1. (ISO 7000-2499) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Sterile |
Indicates a medical device that has been subjected to a sterilization process |
 |
ISO 15223-1
Reference no. 5.2.2. (ISO 7000-2500) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Sterilized using aseptic processing techniques |
Indicates a medical device that has been manufactured using accepted aseptic techniques |
 |
ISO 15223-1
Reference no. 5.2.3. (ISO 7000-2501) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Sterilized using ethylene oxide |
Indicates a medical device that has been sterilized using ethylene oxide. |
 |
ISO 15223-1 Reference no. 5.2.4
(ISO 7000-2502) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Sterilized using irradiation |
To indicate that the device is provided sterile and has been sterilized using irradiation. |
 |
ISO 15223-1 Reference no. 5.2.5
(ISO 7000-2503) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Sterilized using steam or dry heat |
To indicate that the device is provided sterile and has been sterilized using steam or dry heat. |
 |
ISO 15223-1 Reference no. 5.2.9
(ISO 7000-3084) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Sterile fluid path |
To identify the presence of a sterile fluid path within the medical device when other parts of the medical device are not necessarily supplied sterile. |
 |
ISO 15223-1
Reference no. 5.2.6. (ISO 7000-2608) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Do not resterilize |
Indicates a medical device that is not to be resterilized. |
 |
ISO 15223-1 Reference no. 5.2.7
(ISO 7000-2609) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Non-sterile |
To indicate that the device that is normally provided sterile in the same or similar packaging has not been sterilized. |
 |
ISO 15223-1
Reference no. 5.2.8. (ISO 7000-2606) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Do not use if package is damaged |
To indicate that the device must not be used if the package holding the device is damaged, for example on packaging of medical devices. |
 |
ISO 15223-1
Reference no. 5.2.11.
(ISO 7000-3707) |
Medical Devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. |
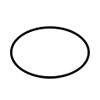
Single sterile barrier system |
Indicates a single sterile barrier system. |
 |
ISO 15223-1 Reference no.
(ISO 7000-3704) |
Medical Devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. |
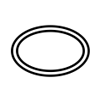
Double sterile barrier system |
Indicates two sterile barrier systems. |
 |
ISO 15223-1
Reference no. 5.2.13.
(ISO 7000-3708) |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements. |
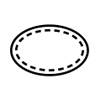
Single sterile barrier system with protective packaging inside |
Indicates a single sterile barrier system with protective packaging inside. |
 |
ISO 15223-1
Reference no.
(ISO 7000-3709) |
Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements. |
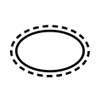
Single sterile barrier system with protective packaging outside |
To indicate that there is a single sterile barrier system with protective packaging outside. |
 |
ISO 7000 / IEC 60417
(ISO 7000-6043) |
IEC 60417 — Graphical Symbols for Use on Equipment |
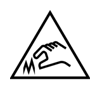
Caution, sharp edges |
To indicate that the marked item contains sharp edges and should not be touched without taking care |
 |
ISO 15223-1
Reference no. 5.4.1. (ISO 7000-0659) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Biological risks |
To indicate a reference to substances that may be hazardous to men, animals, plants, or the environment based on biological activity (for example, holding a virus) |
 |
IEC 60601-1 Ref no. Table D.2, Symbol 20
(ISO 7000 / IEC 60417-5333) |
IEC 60417 — Graphical Symbols for Use on Equipment |
Type BF applied part |
To identify a type BF applied part complying with IEC 60601-1. |
 |
ISO 7000 / IEC 60417
(ISO 7000-6414) |
IEC 60417 — Graphical Symbols for Use on Equipment |
WEEE; waste electrical and electronic equipment; crossed-out wheeled bin |
To indicate that separate collection for waste electric and electronic equipment (WEEE) is required. |
 |
ISO 15223-1
Ref no. 5.4.5.
Contains XXX (ISO 7000- 2025) |
Medical devices ‐ Symbols to be used with medical device labels, labeling and information to be supplied |
Contains or presence of XXX |
On medical devices: to indicate that the equipment contains the identified product or substance. |
 |
ISO 7000 / IEC 60417
(ISO 7000 - 0622) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. |
Use no hooks |
To indicate that hooks shall not be used for handling the transport package. |
 |
ISO 15223-1
Reference no. 5.7.8. (ISO 7000-3728) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. |
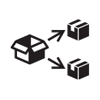
Translation |
To identify that the original medical device information has undergone a translation which supplements or replaces the original information. |
 |
ISO 15223-1
Reference no. 5.7.9. (ISO 7000-3727) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. |
Repackaging |
To identify that a modification to the original medical device packaging configuration has occurred. |
 |
EC 60601-1 Reference no. Table D.2, Safety sign 10 (ISO 7010-M002) |
Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance |
Refer to instruction manual/booklet |
To signify that the instruction manual/booklet must be read. |
 |
ISO 780:1997
Reference no. 0623
(ISO 7000-0623) |
Graphical symbols for use on equipment - registered symbols |
This way up |
To indicate correct upright position of the transport package. |
 |
IEC-TR-60878
Reference no. 1135
ISO 7000-1135 |
Graphic symbols for use on electrical equipment in a medical practice |
General symbol for
recover/recyclable |
To indicate that the marked item or its material is part of a recovery or recycling process |
 |
N/A |
N/A |
Canadian and US Certification mark |
Products bearing this mark have been tested and certified in accordance with applicable US and Canadian electrical safety and performance standards |
 |
N/A |
N/A |
Prop 65 Warning |
Intended to help Californians make informed decisions about their exposures to specific chemicals |


















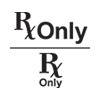







































 International
International
 Brasil
Brasil Danmark
Danmark Deutschland
Deutschland España
España France
France Italia
Italia 日本
日本 Nederland
Nederland Norge
Norge Suomi
Suomi Sverige
Sverige United States
United States United Kingdom
United Kingdom



