Notre site est réservé aux professionnels de santé.
Cataracte
Découvrez notre gamme de lentilles intraoculaires, d'équipements et d'instruments pour vos chirurgies de la cataracte.
Glaucome
BVI fournit un traitement du glaucome polyvalent combiné à des accessoires de qualité pour répondre à vos besoins.
Vitréo-rétinienne
Vos procédures vitréo-rétiniennes soutenue par nos solutions innovantes et nos fluides chirurgicaux.
Autres
Nous soutenons vos procédures de cornée, sécheresse oculaire, lacrymale, oculoplastie et réfractives.


BVI® place l’avenir de la vision au cœur de ses préoccupations.
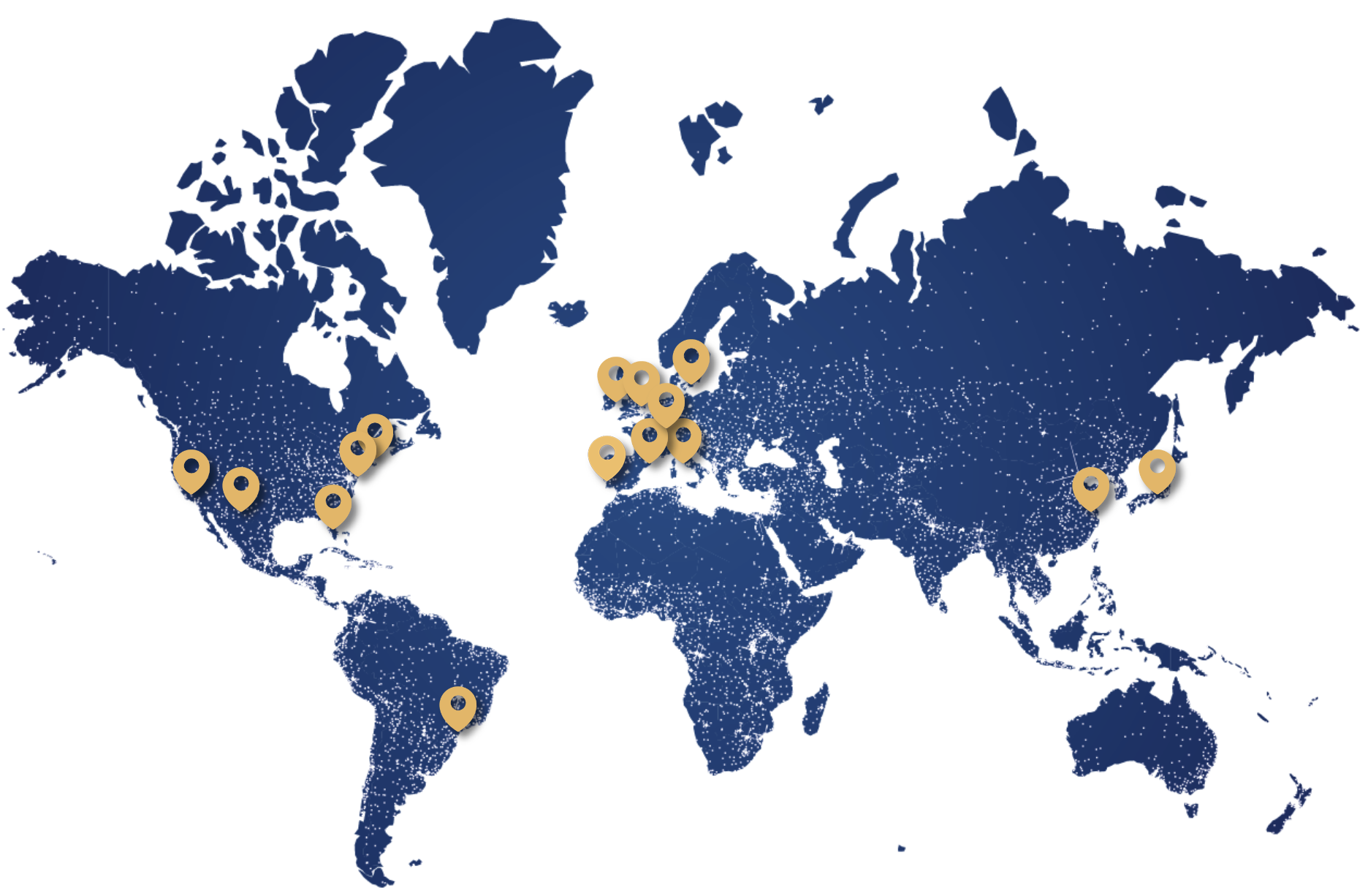
Présente dans plus de 90 pays, BVI est l’une des entreprises de chirurgie ophtalmique diversifiées à la croissance la plus rapide au monde.
Notre objectif : améliorer la vie de millions de patients atteints d’affections telles que la cataracte, les troubles réfractifs, le glaucome, les pathologies rétiniennes et la sécheresse oculaire. Nous avons développé notre stratégie autour d’un concept simple: être fiers de fournir des solutions innovantes à nos médecins et à nos patients, basées sur leurs besoins.


Rejoignez notre équipe
Chez BVI, nous recherchons des professionnels engagés, innovants et talentueux qui souhaitent développer leur carrière au sein de l'industrie ophtalmique.
Découvrez nos offres d'emploi

 International
International
 Brasil
Brasil Danmark
Danmark Deutschland
Deutschland España
España France
France Italia
Italia 日本
日本 Nederland
Nederland Norge
Norge Suomi
Suomi Sverige
Sverige United States
United States United Kingdom
United Kingdom